Orange Book Pharmacy Codes
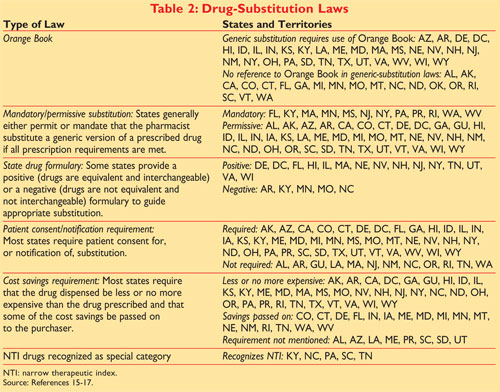
Most pharmacists already know that the orange book, created in 1980 and now in its 28th edition, is an fda publication that lists many drug products and contains indications as to whether generic versions of medications are considered to be equivalent to the drugs manufactured by the innovator company and most often marketed with brand names. Orange book code yes yes yes no no orange book standard flag no yes yes no no reused ndc flag
Optimizing Generic Medication Use
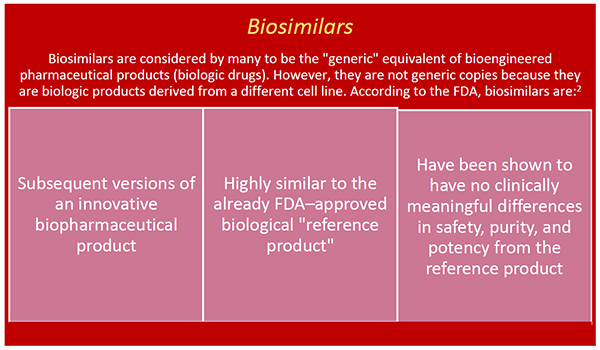
On march 23, 2020, fda removed from the orange book the listings for biological products that have been approved in applications under section 505 of the fd&c act because these products are no longer listed drugs (see section 7002 (e) (4) of the biologics price.

Orange book pharmacy codes. Approved drug products with therapeutic equivalence evaluations. Code b product that fda at this time, considers not to be therapeutically equivalent to other pharmaceutically equivalent products • b*: Modern unixes are roughly c2.
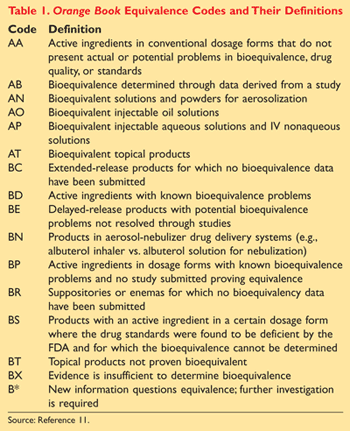
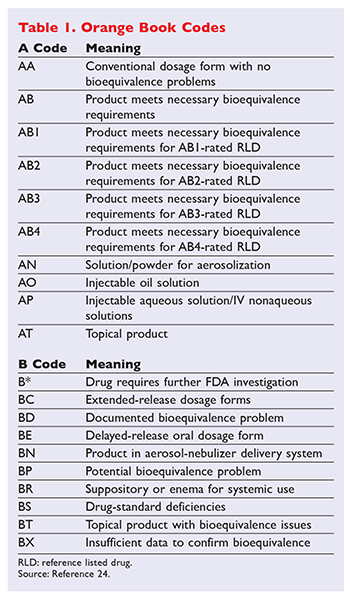
10.15406/mojbb.2015.01.00013 table 1 summary of fda’s orange book therapeutic equivalence codes code interpretation aa no bioequivalence problems in conventional. Their guides are available by subscription or individual purchase. The second letter (a, b, c, d, e, n, o, p, r, s, t or x).
1xecutive agencies, non departmental public bodies and non ministerial departments. A guide to community pharmacist. Various codes and their interpretations are described in table 1.
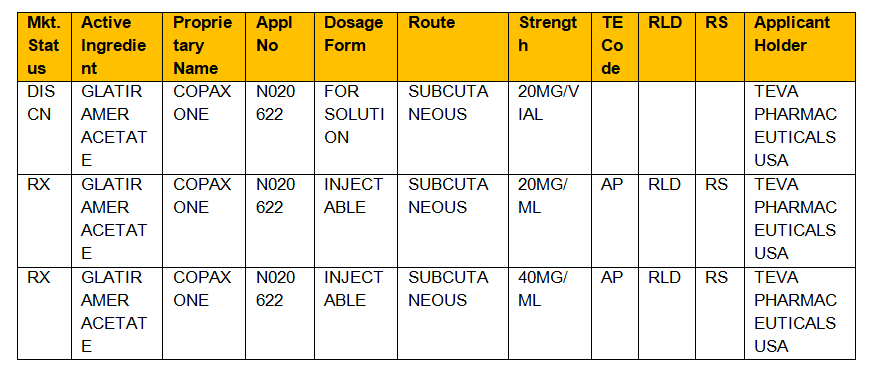
Search approved drug products by active ingredient, proprietary name, applicant, application number, dosage form, route of administration or patent number. Fda’s orange book and ab ratings of pharmaceutical drug products: E 2xelos is a company part owned by the uk government.
Support generic substitution and formulary management by accessing codes and pricing information for drugs that share the same active ingredient, route, strength, dose form and therapeutic efficacy. The orange book | introduction. The evaluations have been prepared as a resource for state health agencies, physicians, pharmacists, and the public to promote public education in the area of drug product selection, as well as to.
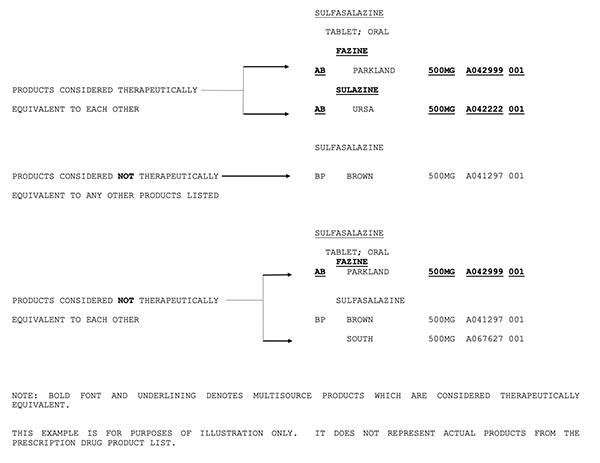
The orange book contains therapeutic equivalence evaluations for approved multisource prescription drug products (usually referred to as generics). 17 in previous editions of the orange book, fda provided a chart outlining therapeutic equivalence codes for all.025 mg levothyroxine sodium drug products in the active section of the orange book. The orange book has been revised.
Search the orange book database. Codes beginning with ‘a’ signify the product is deemed therapeutically equivalent to the reference product for the category. Approved drugs in orange book states such as new york.
Codes beginning with ‘a’ signify the product is deemed therapeutically equivalent to the reference product for the category. Corporate governance code [2] requirements, and overseeing the preparation of the governance. Drug products requiring further fda investigation and review.
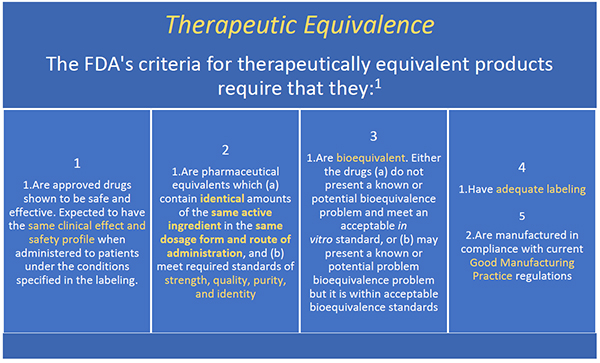
According to the orange book, “a” codes denote “[d]rug products that are considered to be therapeutically equivalent to other pharmaceutically equivalent products.” 4 on the other hand, a “b” designation signifies “[d]rug products that fda, at this time, considers not to be therapeutically equivalent to other pharmaceutically equivalent products.” 4 the second. An a designation means that the fda considers the drug to be the therapeutic equivalent of another pharmaceutically equivalent drug.
Orange Book And Its Applications - Legal Advantage
Generic-substitution Laws

Pharmacology4theditionpdfebook Thebookisapdfebook Onlythereisnoaccesscode Itwillbesenttoth Pharmacology Pharmacy Books Ebook Pdf

Pharmaceutical Press - Rules And Guidance For Pharmaceutical Manufacturers And Distributors 2017 The Orange Guide 2017

Pin On Amazing Books
Module 9 Generic Drugs And Therapeutic Equivalence

50 Off Nanotechnology Law And Guidelines A Practical Guide For The Nanotechnology Industries In Europe By In 2021 Nanotechnology Biomedical Engineering Guidelines

Orange Book And Its Applications - Legal Advantage

Oxford Handbook Of Emergency Medicine Emergency Medicine Emergencymedicinebookspdf Medical Books Free Down Emergency Medicine Medical Textbooks Medicine

A New Social History Of Pharmacy Pharmaceuticals Festival - American Institute Of The History Of Pharmacy
Insights Into Effective Generic Substitution

Generic-substitution Laws

Food And Drug Administration Fda Us Government Bookstore
Module 9 Generic Drugs And Therapeutic Equivalence

Pocket Medicine The Massachusetts General Hospital Handbook Of Internal Medicine 6th Edition Pdf Medicine Book Medical Textbooks Massachusetts General Hospital
Module 9 Generic Drugs And Therapeutic Equivalence

For Data On The Hardcover Edition Please Click Here For Data On The Spanish Language Version Please Click Here Emergency Care Emergency Medical Orange Book
Generic-substitution Laws

Paradigm Publishing Inc 3 Drug Development Chapter Topics Definition Of Drug Significant Drug Discoveries Sources Of Drugs Drug Classifications - Ppt Download